FDA database that tracks heart device harms may miss red flags, safety experts warn
FDA database that tracks heart device harms may miss red flags, safety experts warn

FDA database that tracks heart device harms may miss red flags, safety experts warn
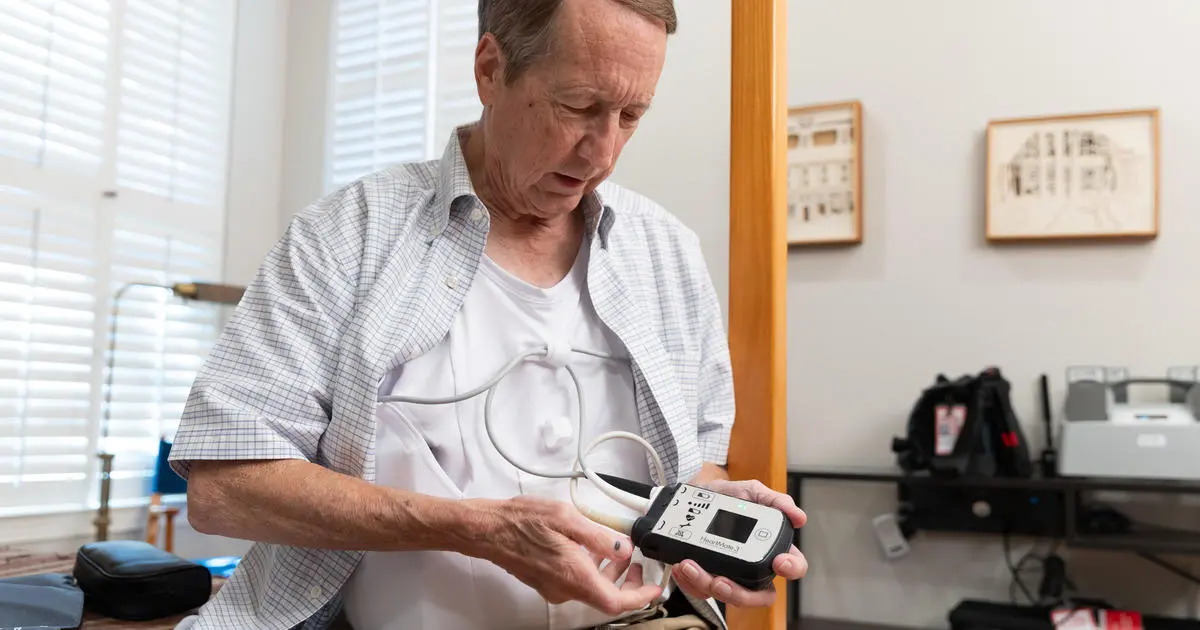
Too old and too sick for a heart transplant, Arvid Herrman was given a choice: Have a mechanical pump implanted in his heart, potentially keeping him alive for several years, or do nothing and almost certainly die within a year.
The 68-year-old Wisconsin farmer chose the pump, called a HeartMate 3 — currently the only FDA-approved device of its kind in use. Instead of extending his life, though, the device led to his death, according to a lawsuit filed in December 2020 by his daughter Jamie Edwards.
The lawsuit alleged that Herrman died because a defect in the locking mechanism of the HeartMate 3 prevented the device from sealing, causing multiple strokes and leading to a severe brain injury and multiorgan failure. Herrman "could not have anticipated the danger this defect … created for him," the lawsuit said.